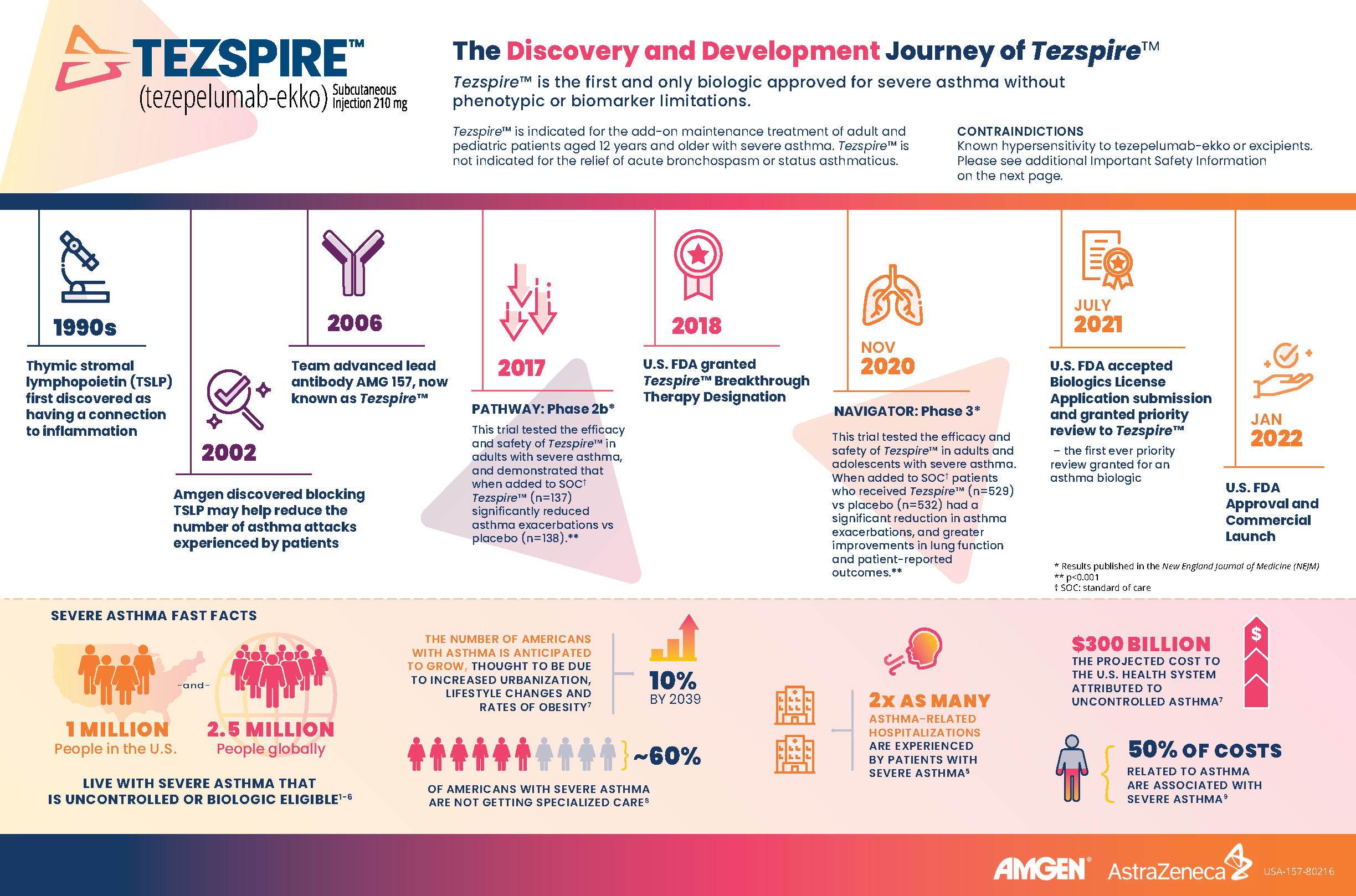
Here are some fast facts about severe asthma and the role of thymic stromal lymphopoietin (TSLP):
- 1 million people in the U.S. and 2.5 million people globally live with severe asthma that is uncontrolled or biologic eligible.1-6
- The number of Americans with asthma is anticipated to grow 10% by 2039, thought to be due to increased urbanization, lifestyle changes and rates of obesity.7
- About 60% of Americans with severe asthma are not getting specialized care.8
- 2x as many asthma-related hospitalizations are experienced by people with severe asthma.5
- Thymic stromal lymphopoietin (TSLP) is a key epithelial cytokine that sits at the top of the inflammatory cascade and acts as an alarmin for the immune system.9
- Amgen discovered blocking TSLP may help reduce the number of asthma attacks experienced by patients after it was first discovered that TSLP had a connection to inflammation in the 1990s.
- Amgen and AstraZeneca jointly developed Tezspire™, the first and only biologic approved for severe asthma without phenotypic or biomarker limitations.9-18
INDICATION
TEZSPIRE is indicated for the add-on maintenance treatment of adult and pediatric patients aged 12 years and older with severe asthma.
TEZSPIRE is not indicated for the relief of acute bronchospasm or status asthmaticus.
IMPORTANT SAFETY INFORMATION
CONTRAINDICATIONS
Known hypersensitivity to tezepelumab-ekko or excipients.
WARNINGS AND PRECAUTIONS
Hypersensitivity Reactions
Hypersensitivity reactions (e.g., rash and allergic conjunctivitis) can occur following administration of TEZSPIRE. These reactions can occur within hours of administration, but in some instances have a delayed onset (i.e., days). In the event of a hypersensitivity reaction, initiate appropriate treatment as clinically indicated and then consider the benefits and risks for the individual patient to determine whether to continue or discontinue treatment with TEZSPIRE.
Acute Asthma Symptoms or Deteriorating Disease
TEZSPIRE should not be used to treat acute asthma symptoms, acute exacerbations, acute bronchospasm, or status asthmaticus.
Abrupt Reduction of Corticosteroid Dosage
Do not discontinue systemic or inhaled corticosteroids abruptly upon initiation of therapy with TEZSPIRE. Reductions in corticosteroid dose, if appropriate, should be gradual and performed under the direct supervision of a physician. Reduction in corticosteroid dose may be associated with systemic withdrawal symptoms and/or unmask conditions previously suppressed by systemic corticosteroid therapy.
Parasitic (Helminth) Infection
It is unknown if TEZSPIRE will influence a patient's response against helminth infections. Treat patients with pre-existing helminth infections before initiating therapy with TEZSPIRE. If patients become infected while receiving TEZSPIRE and do not respond to anti-helminth treatment, discontinue TEZSPIRE until infection resolves.
Live Attenuated Vaccines
The concomitant use of TEZSPIRE and live attenuated vaccines has not been evaluated. The use of live attenuated vaccines should be avoided in patients receiving TEZSPIRE.
ADVERSE REACTIONS
The most common adverse reactions (incidence ≥3%) are pharyngitis, arthralgia, and back pain.
USE IN SPECIFIC POPULATIONS
There are no available data on TEZSPIRE use in pregnant women to evaluate for any drug-associated risk of major birth defects, miscarriage, or other adverse maternal or fetal outcomes. Placental transfer of monoclonal antibodies such as Tezepelumab-ekko is greater during the third trimester of pregnancy; therefore, potential effects on a fetus are likely to be greater during the third trimester of pregnancy.
Please see the Tezspire full Prescribing Information.
You may report side effects related to AstraZeneca products by clicking here.
References:
- Centers for Disease Control and Prevention. Available at: https://www.cdc.gov/asthma/most_recent_national_asthma_data.htm. Accessed November 2021.
- Kupczyk M, Wenzel S. J Intern Med 2012;272:121–32.
- Wenzel S. Am J Respir Crit Care Med. 2005;172;149–60.
- Chung KF, et al. Eur Respir J. 2014; 43: 343–73.
- Chastek, et al. J Manag Care Spec Pharm 2016;22:848-861.
- Chen, et al. Curr Med Res Opin 2018;34(12):2075-2088.
- American Thoracic Society. Available at: https://www.thoracic.org/about/newsroom/press-releases/journal/2019/uncontrolled-asthma-over-next-20-years-likely-toadd-300-billion-to-us-health-care-bill.php. Accessed November 2021.
- Most, J. F. The Journal of Allergy and Clinical Immunology: In Practice, 9(10). https://doi.org/10.1016/j.jaip.2021.05.003
- Corren J, et al. N Engl J Med. 2017; 377: 936-946.
- Menzies-Gow A, et al. N Engl J Med. 2021;384:1800-1809. DOI: 10.1056/NEJMoa2034975.
- Hanania NA, et al. Ann Intern Med. 2011;154 (9): 573-82.
- Yancey SW, et al. Respir Med. 2019; 151: 139-141.
- FitzGerald JM, et al. Lancet Respir Med. 2018; 6 (1): 51-64.
- Castro M, et al. N Engl J Med. 2018; 378 (26): 2486-2496.
- Ortega HG, et al; on behalf of the MENSA Investigators. N Engl J Med. 2014;371(13):1198-207.
- Bleecker ER, et al, on behalf of the SIROCCO study investigators. Lancet 2016: 388 (10056): 2115-2127.
- FitzGerald JM, et al, on behalf of the CALIMA study investigators. Lancet. 2016: 388(10056): 2128-2141.
- Wenzel S, et al. Lancet. 2016 Jul 2;388(10039):31-44


