Biosimilars Development and Manufacturing
Developing a biosimilar is an incredibly complex process. Biosimilars, like all biologics, are produced through an intricate, multistep process, using living cells. The technology we're using to develop and manufacture biologics today is more precise, more accurate, and more quantitative than in years past – giving us the ability to look at multiple attributes to bring reliable, high-quality medicines to patients.1-4,7
End-to-End Biologics Experts: Our Process
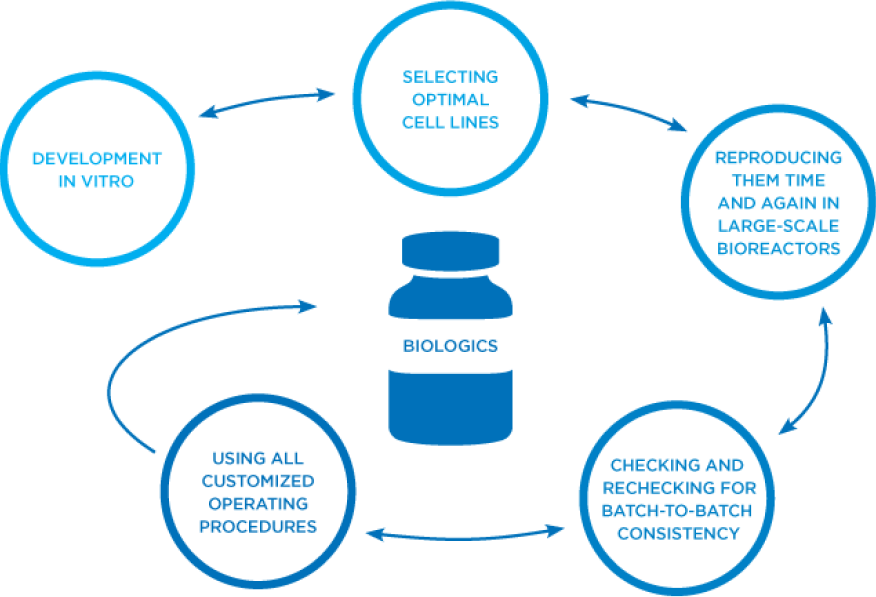
At Amgen, we excel at the highly specialized, iterative process of developing monoclonal antibodies in vitro, scaling up the optimal cell line, time and again, in large-scale bioreactors, and checking and rechecking for batch-to-batch consistency.2,6
Regulatory Approval Pathways
In addition to the safeguards put in place during development in countries with robust regulatory standards, such as the U.S., the approval pathway for biosimilars is rigorous and scientifically appropriate. Approval of a biosimilar is based on the totality of evidence.4 This may include:
- Comparative analytical characterization
- Comparative nonclinical studies
- Comparative clinical pharmacology testing
- Additional comparative clinical studies
Additionally, pharmacovigilance is essential for all biologics, including biosimilars, to protect patients. Pharmacovigilance tracks adverse events and attributes them to the correct product and manufacturer. The ability to identify a biological medicine through systems that contribute to pharmacovigilance is a critical step in promoting patient safety.5
A Strong and Secure Supply Chain
Gaps in supply can have serious consequences, leaving patients without the medicines they need. Amgen's nearly unmatched supply reliability record promotes uninterrupted and reliable supply of high-quality biosimilar medicines to every patient.7
Manufacturers of biologic medicines are responsible for monitoring all steps during product development and manufacturing to ensure that the medicine is pure, has the desired strength, and is stable.2 A reliable supply chain is key to this responsibility. Biosimilar supply chains should:
- Ensure product authentication
- Maintain strict warehouse security
- Incorporate vigilant cargo security
- Conduct market surveillance
References:
- Sekhon, et al. Biosimilars. 2011:1,1-11
- Desanvicente-Celis, et al. Immunotherapy. 2012;4:1841-1857.
- Mellstedt H, Niederwieser D, Ludwig H. The challenge of biosimilars. Ann Oncol. 2008;19:411-419.
- USFDA. Scientific considerations in demonstrating biosimilarity to a reference product. 2015.
- Felix, et al. Nat Biotechnol. 2014;32:128-129.
- Ramanan S, Grampp G. Drift, evolution, and divergence in biologics and biosimilars manufacturing. BioDrugs.2014;28:363-372.
- One Common Mission to Serve Patients: Amgen Operations Shine with U.S. Biosimilar Launch. https://wwwext.amgen.com/stories/2023/01/one-common-mission-to-serve-patients---amgen-operations-shine-with-us-biosimilar-launch. Accessed January 1, 2024.
