Comprehensive biomarker testing can play an important role in the personalized treatment for patients with non-small cell lung cancer (NSCLC). Research has shown that people living with NSCLC who undergo biomarker testing and receive genotype-directed targeted therapy have a 31% reduced risk of death and an improved survival duration compared to patients with an identified oncogenic mutation who did not receive targeted therapy.1 This is why it's vital to increase accessibility to testing and why Amgen is working with the broader healthcare community to demystify the process and help everyone – from oncologists and nursing staff to the electronic medical record managers and administrative team – to have the information and tools to successfully implement biomarker testing for their patients.
Cancer care teams can face several challenges regarding biomarker testing in the clinical setting. From cost, to inadequate tissue sampling, turnaround time, and communication challenges among care staff and patients – these barriers can impede healthcare providers' ability to treat patients with personalized care.
In a recent event with the Association of Community Cancer Centers (ACCC), I-Fen Chang, vice president, Global Medical Oncology at Amgen, moderated a panel with oncology experts, focusing on the hurdles cancer care teams face when it comes to implementing biomarker testing for patients and what can be done to ease these burdens.
"One of the most stressful times for patients is when they've been told they have cancer and the turnaround time for [biomarker] testing can be much longer than a patient can stand and sometimes much longer than a physician can stand," said Dr. Gerard Silvestri, Professor of Thoracic Oncology, Medical University of South Carolina. "There is sometimes a real lack of clear communication between the pulmonologist, pathologist and oncologist treating the patient. Having that triad of physicians meet and talk about when they're going to test, who they're going to test, how they're going to prepare the specimen, who they're going to send it to, and how they're going to manage the results of that is critically important."
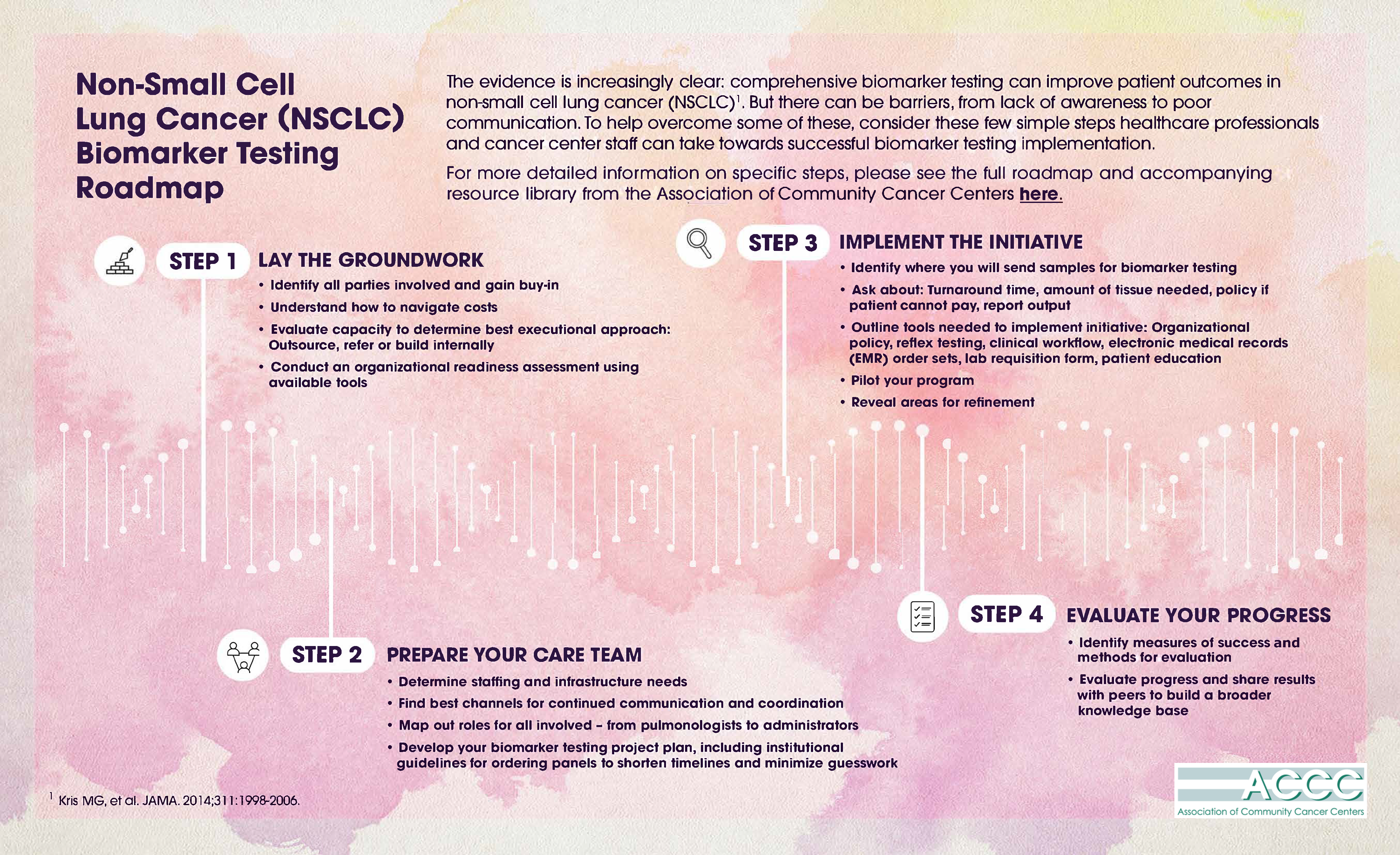
To help overcome some of these barriers and work towards needed solutions, the Association of Community Cancer Centers has created a roadmap outlining simple steps cancer center staff can take towards successful and sustained biomarker testing implementation. Increasing accessibility to testing will give patients and their care team helpful guidance when deciding what treatment might be right for them. For more detail information on specific steps, please see the full roadmap from the Association of Community Cancer Centers here. The content references are provided by an independent, non-profit organization that may provide useful information and resources. Amgen has no control over these programs and provides information as a courtesy only.
And if you or a loved one is interested in learning more about the benefits of biomarker testing, click here. Amgen also provides a suite of educational resources on biomarker testing for HCPs and patients. Be sure to check out the latest video presented by Dr. Harry Hwang that discusses the most common barriers to biomarker testing and how to overcome them.
References:
- Kris MG, et al. JAMA. 2014;311:1998-2006.



