Imagine waking up unable to smell your morning coffee or breathe easily through your nose. For people living with chronic rhinosinusitis with nasal polyps (CRSwNP), these aren't occasional annoyances—they're daily realities.1,2 Though many may not recognize the term nasal polyps, CRSwNP affects approximately 1.1% of adults in the U.S.3 and up to 320 million people worldwide.4,5 As many as 80% of people with CRSwNP can experience olfactory dysfunction, a significant indicator of Type 2 inflammation and disease severity.6
Despite the burden of this disease, symptoms are often minimized or misdiagnosed.1,7 Even after a correct diagnosis, current management options may still present clinical challenges.8 While intranasal corticosteroids (INCS) or surgery can lead to some improvements, as nasal polyp recurrence is a possibility, systemic corticosteroids (SCS) or revision surgery may still be needed for some patients.9 In an Italian survey of 437 physicians capturing current trends in the use of local and SCS in patients with CRSwNP, approximately 70% of physicians said that SCS provides only temporary symptom relief.9 And, in a survey of 389 patients with CRSwNP, 80% reported uncontrolled and partly controlled symptoms within 3 to 5 years after surgery.10
Standard of Care in the Treatment of CRSwNP
Current CRSwNP treatment typically begins with intranasal corticosteroids and saline rinses.8 As the disease worsens, many patients require repeated oral corticosteroid (OCS) courses and/or multiple sinus surgeries.8 In fact, studies show that patients can experience regrowth of polyps within just a few years of surgery. Despite surgical intervention, approximately 40% of patients with CRSwNP remain inadequately controlled.10
Additionally, studies have shown that prolonged use of oral corticosteroids can lead to adverse effects including cardiovascular, metabolic and gastrointestinal disorders,11 further highlighting the need for additional treatment options in this patient population.
Airway Disease Research: From Symptoms to Inflammation to Epithelium
The history of airway disease research has been one of progressive discovery. For example, asthma research for decades was shaped by the Physiological Era, proving the effectiveness of early therapies like bronchodilation and later, oral corticosteroids.12 The development of corticosteroids ushered in the Immunological Era, an age of asthma diagnosis and management based on inflammatory markers.12
Later treatment options for respiratory diseases expanded to include intranasal corticosteroids, surgery, and biologics.12 These innovations helped many and paved the way for a deeper understanding of specific drivers of disease.
Today, research suggests that we have entered a new frontier in the understanding of the pathophysiology of airway diseases: the Epithelial Era.12
Why the Epithelium Matters
Emerging science has transformed how we understand the fundamental role of the airway epithelium, not just in asthma but in other respiratory diseases as well—including chronic rhinosinusitis with nasal polyps. CRSwNP is now recognized as part of a broader systemic inflammatory process that begins at the airway epithelium.12
Far from being a simple barrier, the epithelium is an active immune organ. It lines the respiratory tract, defending against allergens, pollutants, bacteria and viruses.12
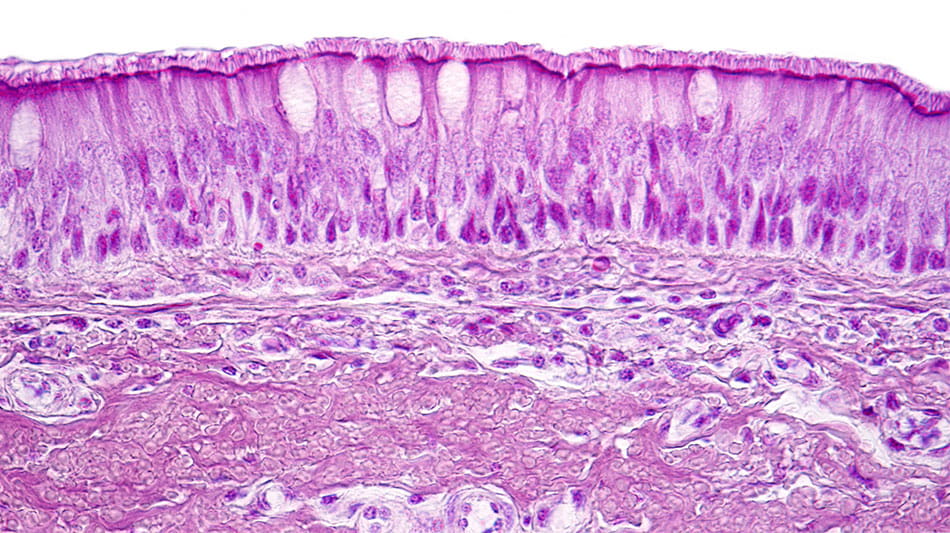
Light micrograph showing the respiratory epithelium. This protective tissue lines the respiratory tract, acting as both barrier and immune sentinel against pathogens and irritants.12
In people with CRSwNP, this protective barrier can become compromised and mounting evidence points to impairment of the epithelial barrier as playing an important role in disease pathophysiology.13-16 When that happens, the epithelium releases “alarmin” cytokines such as thymic stromal lymphopoietin (TSLP), IL-33 and IL-25, which set off a chain reaction of inflammation that can fuel polyp formation and chronic symptoms.13-16
Connecting the Upper and Lower Airways
Many patients with CRSwNP also experience comorbid severe asthma or other lower-airway inflammatory conditions.17 Increasing evidence points to shared epithelial inflammation-driven mechanisms across the upper and lower airways, reinforcing the importance of therapies that can help address both.15,17-18
The recognition of epithelial dysfunction as a possible central driver of airway disease could offer a new way of thinking about respiratory research and care.12,15
Targeting TSLP in CRSwNP
Although the mechanism of action in CRSwNP has not been definitively established, by blocking TSLP it's possible to help prevent the activation of multiple drivers,15 offering a novel approach for patients who have long relied on steroids or surgery.12 TEZSPIRE® (tezepelumab-ekko) is the first and only biologic approved for CRSwNP that targets TSLP as a key source of inflammation.19
TEZSPIRE (tezepelumab-ekko) injection 210 mg is now approved for the add-on maintenance treatment of adult and pediatric patients aged 12 years and older with inadequately controlled chronic rhinosinusitis with nasal polyps (CRSwNP).
TEZSPIRE is also indicated for the add-on maintenance treatment of adult and pediatric patients aged 12 years and older with severe asthma. TEZSPIRE is not indicated for the relief of acute bronchospasm or status asthmaticus.
TEZSPIRE is contraindicated in patients who have known hypersensitivity to tezepelumab-ekko or any of its excipients. Please see additional Important Safety Information below.
TEZSPIRE® (tezepelumab-ekko) Important Safety Information
Contraindications
Known hypersensitivity to tezepelumab-ekko or excipients.
WARNINGS AND PRECAUTIONS
Hypersensitivity Reactions
Hypersensitivity reactions were observed in the clinical trials (eg, rash and allergic conjunctivitis) following the administration of TEZSPIRE. Postmarketing cases of anaphylaxis have been reported. These reactions can occur within hours of administration, but in some instances have a delayed onset (ie, days). In the event of a hypersensitivity reaction, consider the benefits and risks for the individual patient to determine whether to continue or discontinue treatment with TEZSPIRE.
Acute Asthma Symptoms or Deteriorating Disease
TEZSPIRE should not be used to treat acute asthma symptoms, acute exacerbations, acute bronchospasm, or status asthmaticus.
Abrupt Reduction of Corticosteroid Dosage
Do not discontinue systemic or inhaled corticosteroids abruptly upon initiation of therapy with TEZSPIRE. Reductions in corticosteroid dose, if appropriate, should be gradual and performed under the direct supervision of a physician. Reduction in corticosteroid dose may be associated with systemic withdrawal symptoms and/or unmask conditions previously suppressed by systemic corticosteroid therapy.
Parasitic (Helminth) Infection
It is unknown if TEZSPIRE will influence a patient's response against helminth infections. Treat patients with pre-existing helminth infections before initiating therapy with TEZSPIRE. If patients become infected while receiving TEZSPIRE and do not respond to anti-helminth treatment, discontinue TEZSPIRE until infection resolves.
Live Attenuated Vaccines
The concomitant use of TEZSPIRE and live attenuated vaccines has not been evaluated. The use of live attenuated vaccines should be avoided in patients receiving TEZSPIRE.
ADVERSE REACTIONS
The most common adverse reactions (incidence ≥ 3%) are:
- Asthma: pharyngitis, arthralgia, and back pain.
- Chronic rhinosinusitis with nasal polyps: nasopharyngitis, upper respiratory tract infection, epistaxis, pharyngitis, back pain, influenza, injection site reaction and arthralgia
USE IN SPECIFIC POPULATIONS
There are no available data on TEZSPIRE use in pregnant women to evaluate for any drug-associated risk of major birth defects, miscarriage, or other adverse maternal or fetal outcomes. Placental transfer of monoclonal antibodies such as tezepelumab-ekko is greater during the third trimester of pregnancy; therefore, potential effects on a fetus are likely to be greater during the third trimester of pregnancy.
Please see the full Prescribing Information including Patient Information and Instructions for Use.You may report side effects related to AstraZeneca products by clicking here.
References
- Bachert C, Marple B, Schlosser RJ, et al. Adult chronic rhinosinusitis. Nat Rev Dis Primers. 2020;6:86.
- Claeys N, Teeling MT, Legrand P, et al. Patients Unmet Needs in Chronic Rhinosinusitis With Nasal Polyps Care: A Patient Advisory Board Statement of EUFOREA. Front Allergy. 2021;2:761388.
- Palmer JN, Messina JC, Biletch R, Grosel K, Mahmoud RA. A cross-sectional, population-based survey of U.S. adults with symptoms of chronic rhinosinusitis. Allergy Asthma Proc. 2019;40(1):48-56.
- Chen S, Zhou A, Emmanuel B, Thomas K, Guiang H. Systematic literature review of the epidemiology and clinical burden of chronic rhinosinusitis with nasal polyposis. Curr Med Res Opin. 2020;36(11):1897-1911.
- United States Census Bureau. U.S. and World Population Clock website. www.census.gov/popclock/world. Accessed October 3, 2025.
- Gavidia M. Improved Sense of Smell Achieved With Biologics for CRS With Nasal Polyps, Asthma. Am J Manag Care. 2022. www.ajmc.com/view/improved-sense-of-smell-achieved-with-biologics-for-crs-with-nasal-polyps-asthma. Accessed October 3, 2025.
- Hwee J, Lee L, Small M, et al. The chronic rhinosinusitis with nasal polyp patient journey in the United States and Europe. Allergy, Asthma & Clinical Immunology. 2024;20(1):17.
- Fokkens WJ, Lund VJ, Hopkins C, et al. European position paper on rhinosinusitis and nasal polyps 2020. Rhinology. 2020;58(suppl. S29):1-464.
- De Corso E, Pipolo C, Cantone E, et al. Survey on use of local and systemic corticosteroids in the management of chronic rhinosinusitis with nasal polyps: identification of unmet clinical needs. J Pers Med. 2022;12(6):897.
- Van der Veen J, Seys SF, Timmermans M, et al. Real-life study showing uncontrolled rhinosinusitis after sinus surgery in a tertiary referral centre. Allergy. 2017;72(2):282-290.
- Yasir M, Goyal A, Sonthalia S. Corticosteroid Adverse Effects. StatPearls. 2023. www.ncbi.nlm.nih.gov/books/NBK531462. Accessed October 3, 2025.
- Brightling CE, Marone G, Aegerter H, et al. The epithelial era of asthma research: knowledge gaps and future directions for patient care. Eur Respir Rev. 2024;33:240221.
- Akdis CA, Bachert C, Cingi C, et al. Endotypes and phenotypes of chronic rhinosinusitis: A PRACTALL document. J Allergy Clin Immunol. 2013;131(6):1479-90.
- Corren J, Parnes JR, Wang L, et al. Tezepelumab in adults with uncontrolled asthma. N Engl J Med. 2017;377:936-946.
- Laidlaw TM, Menzies-Gow A, Caveney S, et al. Tezepelumab Efficacy in Patients with Severe, Uncontrolled Asthma with Comorbid Nasal Polyps in NAVIGATOR. J Asthma Allergy. 2023;16:915-932.
- Roan F, Obata-Ninomiya K, Ziegler SF. Epithelial cell-derived cytokines: more than just signaling the alarm. The Journal of Clinical Investigation. 2019;129(4):1441-1451.
- Langdon C, Mullol J. Nasal polyps in patients with asthma: prevalence, impact, and management challenges. J Asthma Allergy. 2016;9:45-53.
- Yan B, Lan F, Li J, Wang C, Zhang L. The mucosal concept in chronic rhinosinusitis: Focus on the epithelial barrier. J Allergy Clin Immunol. 2024;153(5):1206-1214.
- Amgen/AstraZeneca. TEZSPIRE® (tezepelumab-ekko) prescribing information. 2025.